Categories
Tags
-
#Aerospace 3D Printing Market Share
#Plant Growth Chambers Market Share
#Nurse Call Systems Market Size
#Cosmetic Dentistry Market Size & Share
#Plant Growth Chambers Market Size
#Packaged Coconut Water Market Share
#Heparin Market Size
#Burn Care Market share
#3D Printing Market Size
#3D Printing Market Trends
#Cosmetics Market Size
#Cosmetics Market Share
#Cosmetics Market Trends
#Anti-Money Laundering (AML) Software Market
#Chemoinformatics Market Size
#Global Eggshell Membrane Market Size
#Audio DSP Market Share
#Pasta Sauce Market share
#Medical Tourism Market Size
#Offshore Support Vessels Industry Overview
#Workwear Market Size
#Autoinjector Market Size
#Autoinjector Market Share
#Autoinjector Market Trends
#Pharmaceutical Filtration Market Share
#Wallpaper Market Share
#South Korea API Market Outlook: Digital Integration
#South Korea Forage Market Size
#South Korea Forage Market share
#South Korea Forage Market Trends
#Battery Electrolyte Market Size
#South Korea Advertising Market Outlook
#South Korea Advertising Market share
#South Korea Advertising Market growth
#Plant Growth Chambers Market Trends
#Cosmetic Dentistry Market 2025: Size
#Cosmetic Dentistry Market Trends
#Rhinoplasty Market share
#Rhinoplasty Market size
#Rhinoplasty Market Trends
#Dietary Fiber Market Size
#South Korea Drones Market Report
#South Korea Drones Market share
#South Korea Drones Market Trends
#Marine Plywood Market Size
#Marine Plywood Market Share
#Marine Plywood Market Trends
#Structural Core Materials Market Size
#Recommendation Engine Market Share
#Coconut Water Market Size
#Coconut Water Market Trends
#South Korea Electric Vehicle Market share
#South Korea Electric Vehicle Market Report 2025–2033: Size
#South Korea Electric Vehicle Market Trends
#Microgreens Market Size
#Microgreens Market Share
#Microgreens Market Trends
#Global Mezcal Market Size
#Cotton Yarn Market Size
#Global Virtual Data Room Market share
#Global Virtual Data Room Market size
#Global Virtual Data Room Market Trends
#Sustainable Athleisure Market Size
#Sustainable Athleisure Market share
#Sustainable Athleisure Market Trends
#Coffee Roaster Market Analysis
#Coffee Roaster Market share
#Coffee Roaster Market size
#High-End Lighting Market share
#High-End Lighting Market Size
#High-End Lighting Market Trends
#Fire Alarm and Detection System Market Size
#Fire Alarm and Detection System Market share
#Fire Alarm and Detection System Market Trends
#Lung Cancer Therapeutics Market Size
#Lung Cancer Therapeutics Market share
#Lung Cancer Therapeutics Market Trends
#3D printing materials market Size
#3D printing materials market share
#3D printing materials market Trends
#Cashew Milk Market Size
#Cashew Milk Market share
#Cashew Milk Market Trends
#jewellery market share
#jewellery market size
#jewellery market Trends
#Energy Drinks Market share
#Energy Drinks Market size
#Energy Drinks Market Trends
Archives
Pharmaceutical Filtration Market Share, Size, In-Depth Analysis
-
IMARC Group, a leading market research company, has recently released a report titled "Pharmaceutical Filtration Market Size, Share, Trends and Forecast by Product, Technique, Application, Scale of Operation, and Region, 2025-2033" The study provides a detailed analysis of the industry, including the global pharmaceutical filtration market size, share, growth, trends, and forecast. The report also includes competitor and regional analysis and highlights the latest advancements in the market.
Pharmaceutical Filtration Market Overview
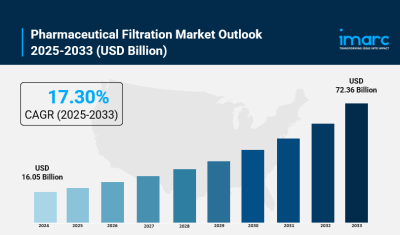
- Pharmaceutical Filtration Market Size: Valued at USD 16.1 Billion in 2024.
- Pharmaceutical Filtration Market Forecast: The market is expected to reach USD 72.4 billion by 2033, growing at 18.2% annually.
- Market Growth: The pharmaceutical filtration market is experiencing robust growth driven by increasing demand for biopharmaceuticals, rising prevalence of chronic diseases, and stringent regulatory standards for drug purity.
- Technology Integration: Continual technological advancements in filtration technologies, including single-use systems, membrane filters, and depth filtration, are revolutionizing pharmaceutical manufacturing processes.
- Product Innovation: Membrane filters dominate the market, with innovations in MCE, PTFE, PVDF, and nylon membrane filters enabling precision sterilization, particle removal, and drug purification.
- Regulatory Compliance: Growing emphasis on cGMP manufacturing practices and regulatory compliance is driving investments in advanced filtration equipment and technologies.
- Key Players: Industry leaders include 3M Company, Danaher Corporation, Merck KGaA, Sartorius AG, Parker Hannifin Corporation, and Eaton Corporation, which provide cutting-edge filtration solutions.
- Market Challenges: High initial investment costs, complex validation processes, and the need for specialized training present ongoing challenges for market participants.
Request Your Free “Pharmaceutical Filtration Market” Insights Sample PDF: https://www.imarcgroup.com/pharmaceutical-filtration-market/requestsample
Our report includes:
- Market Dynamics
- Market Trends and Market Outlook
- Competitive Analysis
- Industry Segmentation
- Strategic Recommendations
Industry Trends and Drivers:
- Explosive Growth in Biopharmaceutical Manufacturing:
The pharmaceutical industry is experiencing a transformative shift toward biologic drugs, including monoclonal antibodies, vaccines, therapeutic proteins, and cell and gene therapies. These advanced treatments are revolutionizing healthcare by addressing complex conditions that traditional small-molecule drugs cannot effectively treat. Biologic manufacturing requires exceptionally stringent filtration processes to ensure product purity, sterility, and safety. According to CDC data, approximately 129 million Americans have at least one chronic disease, with 42% experiencing two or more conditions. This creates massive demand for innovative therapies that rely heavily on sophisticated filtration technologies throughout production. Companies are increasingly adopting single-use filtration systems to manage economic pressures and stringent regulatory controls, with recent surveys showing nearly 86% of biopharmaceutical manufacturers using disposable filter cartridges and around 79% using single-use depth filters.
- Revolutionary Single-Use Technology Adoption:
Single-use technologies are transforming biopharmaceutical production and development across the industry. These systems offer critical advantages including higher flexibility to adjust for volume and demand, reduced cross-contamination risks, simplified validation processes, and improved operational efficiency. The shift toward disposable filtration products eliminates time-consuming cleaning and sterilization procedures required for traditional reusable systems, significantly reducing production downtime and contamination risks. For biopharmaceutical manufacturers, single-use systems mean faster changeovers between product batches, lower capital investment requirements, and reduced water and energy consumption. In January 2023, Sartorius and RoosterBio extended their collaboration to develop scalable downstream manufacturing processes for exosome-based therapies, utilizing advanced filtration systems and analytical tools that showcase how single-use technology is enabling next-generation therapeutic development. The growing trend toward personalized medicines further propels single-use systems adoption, as these treatments require flexible manufacturing capabilities that traditional fixed equipment cannot provide.
- Massive Generic Drug Production Expansion:
The global generic drugs market reached 367.1 Billion dollars recently and continues expanding rapidly to meet worldwide healthcare needs. Generic drugs are manufactured in enormous volumes to satisfy global demand, necessitating efficient and scalable filtration processes to ensure quality and regulatory compliance. Every stage of generic drug production—from raw material filtration through final product processing—requires reliable filtration solutions that can handle high throughput while maintaining strict quality standards. The affordability and accessibility of generic medications make them essential for healthcare systems worldwide, particularly in developing countries where cost-effective treatments are critical. As generic drug production scales up globally, pharmaceutical companies are investing heavily in advanced filtration equipment that can process large volumes quickly without compromising product purity or safety. This expansion creates sustained demand for filtration technologies across all pharmaceutical manufacturing scales.
- Rising Chronic Disease Burden Driving Drug Innovation
The increasing prevalence of chronic diseases is fundamentally reshaping pharmaceutical development and manufacturing priorities. Conditions including cancer, diabetes, asthma, COPD, and arthritis affect hundreds of millions of people globally, creating urgent need for diverse medications and treatment options. In December 2023, AstraZeneca Pharma India launched Trastuzumab Deruxtecan (Enhertu) for treating HER2-positive breast cancer, exemplifying how pharmaceutical companies are continuously introducing new medications to address chronic conditions. Each new drug development program requires extensive filtration throughout research, development, clinical trials, and commercial manufacturing phases. The complexity of modern pharmaceuticals, particularly large-molecule biologics, demands increasingly sophisticated filtration solutions capable of handling sensitive compounds while ensuring absolute purity. Water purification, air purification, cell separation, raw material filtration, and final product processing all rely on advanced filtration technologies. As pharmaceutical companies race to develop treatments for chronic diseases, the demand for reliable, scalable, and compliant filtration systems continues accelerating across the entire drug development and manufacturing pipeline.
Pharmaceutical Filtration Market Report Segmentation:
Breakup by Product:
- Membrane Filters (MCE, Coated Cellulose Acetate, PTFE, Nylon, PVDF, Others)
- Prefilters and Depth Media
- Single-Use Systems
- Cartridges and Capsules
- Filter Holders
- Filtration Accessories
- Others
Membrane filters hold the majority market share, critical for precision sterilization and drug purification, with MCE and PTFE filters widely used due to their uniform pore geometry and chemical compatibility.
Breakup by Technique:
- Microfiltration
- Ultrafiltration
- Crossflow Filtration
- Nanofiltration
- Others
Microfiltration dominates with the largest share due to its versatility in removing wide-ranging impurities from biopharmaceutical products without significantly impacting biological activity or stability.
Breakup by Application:
- Final Product Processing
- Raw Material Filtration
- Cell Separation
- Water Purification
- Air Purification
Final product processing accounts for the largest share as it represents the critical final quality control step ensuring pharmaceutical products meet stringent regulatory standards.
Breakup by Scale of Operation:
- Manufacturing Scale
- Pilot-Scale
- Research and Development Scale
Manufacturing scale operations dominate current market share, though the research and development segment is experiencing fastest growth driven by pharmaceutical innovation and new drug development pipelines.
Breakup By Region:
- North America (United States, Canada)
- Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others)
- Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
- Latin America (Brazil, Mexico, Others)
- Middle East and Africa
Who are the key players operating in the industry?
The report covers the major market players including:
- 3M Company
- Amazon Filters Ltd
- Danaher Corporation
- Eaton Corporation plc
- General Electric Company
- Graver Technologies LLC
- Meissner Filtration Products Inc
- Merck KGaA
- Parker Hannifin Corporation
- Sartorius AG
Ask Analyst For Request Customization: https://www.imarcgroup.com/request?type=report&id=4477&flag=E
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provides a comprehensive suite of market entry and expansion services.
IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact US:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:
 +91 120 433 0800
+91 120 433 0800United States: +1–201971–6302